….Thanks, everyone, for joining today. For those that aren't as familiar with KemPharm, I'll just give a brief introduction here, and discuss our value proposition. KemPharm historically has been focused on the discovery and development of prodrugs. We to date have 2 FDA-approved and partnered medications AZSTARYS® and APADAZ® and certainly has been the history of the organization. As we announced back in January, our new focus is on rare disease and CNS drug development looking at innovative therapies, including in this particular case, a prodrug, but we're not subject to that limitation. It's this development of these novel treatments, we believe, will create the largest value for us and our shareholders in the future.
Today's update will actually be relatively short. We did provide an update just last -- about 6 weeks ago as well and have been making great progress on all of the items that we discussed and had planned for this quarter. There are several items that I have in the update here, that we'll be looking forward to scheduling and/or updating as we move forward.
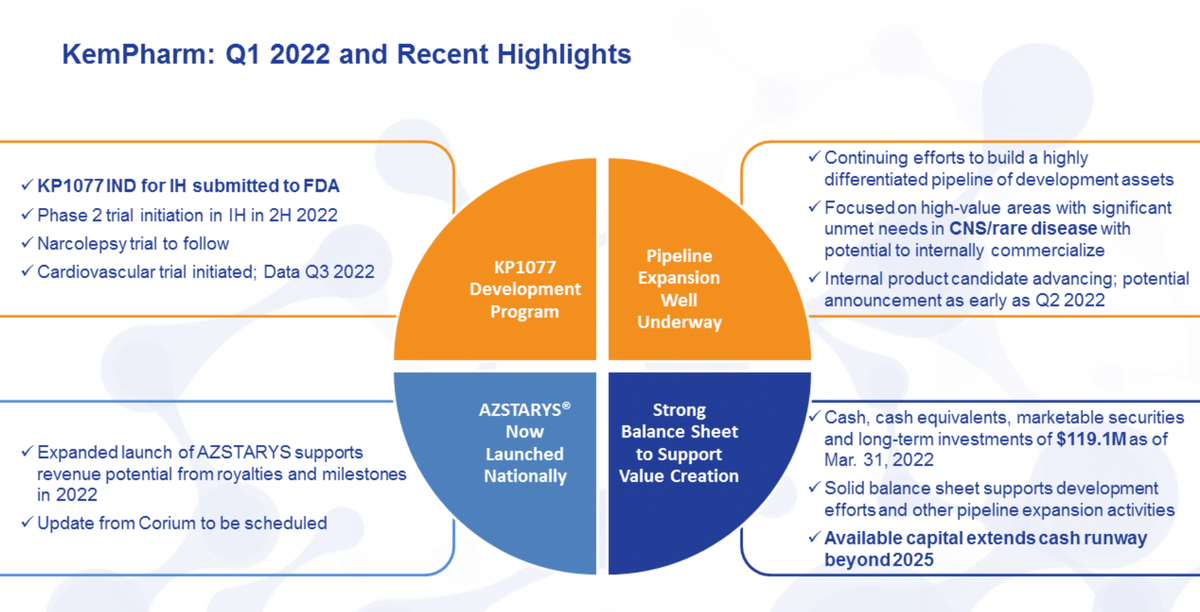
So in the second -- in the first quarter, we accomplished a number of different goals that we had as well as in the early part of the second quarter here. We filed the IND for KP1077 for the treatment of idiopathic hypersomnia as well as got underway with the planning and the trial initiation around the Phase II. That should initiate officially in the second half of this year. Once that study initiates, we'll shortly follow that with the narcolepsy trial. So those 2 will be working almost in parallel at that point.
I think most importantly and with the near-term sort of milestone that we have for KP1077 is our cardiovascular trial. This is looking at the safety of doses used, and I have a little more data here to provide but we actually should have a data readout in that in the third quarter. And this is a key differentiation for that particular product.
And we did provide a little wholesome description of how AZSTARYS is proceeding. Of course, this is our ADHD product partnered with Corium, where they expanded the launch of AZSTARYS. It would support what we still believe is the opportunity to earn milestones and royalties in the continuing part of this year.
I did mention on the last call that we are planning to have an update from Corium. We're still in the planning stages of that. Of course, it's not on the near-term horizon. We're kind of looking at what time ideally works for them as well as trying to find when it may be most effective for them to discuss upcoming school year sales activities.
I did mention our attempt here to move forward and build the pipeline of differentiated assets in the CNS and rare disease space. We have an internal product candidate that we plan to advance into development stage, and we'll be announcing that, more than likely, this quarter. Again, it's an expansion upon some of the internal work we've done, so a little bit earlier in that process. And then LaDuane will be providing us a financial update on this call that includes our strong balance sheet to support all of our efforts and external business development, internal development as well as advancing some of our own candidates.
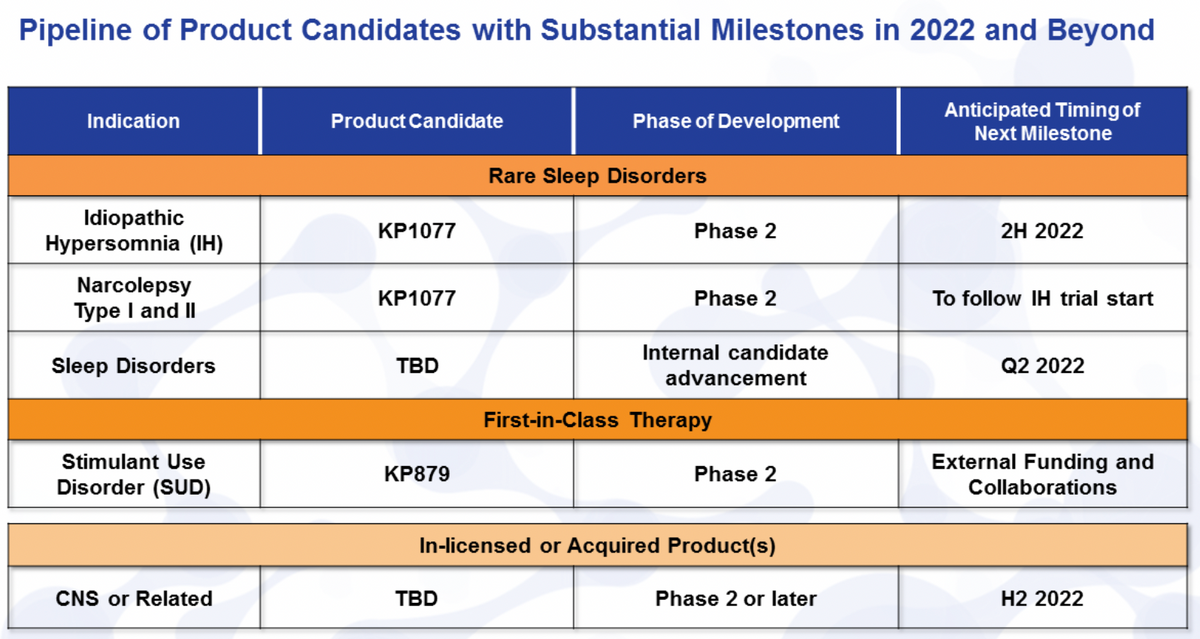
If we look just very quickly here in our pipeline, I've already mentioned KP1077 for the treatment of idiopathic hypersomnia as well as our plans to develop that in narcolepsy. Both of these are rare sleep disorders, many of you may be familiar with. Again, these both have milestones with trial starts the later half of this year and shortly after the idiopathic hypersomnia in the case of narcolepsy. We do have an internal candidate we hope to advance this quarter, and we could have that announcement once we have all of the details around that available to us.
And then as I mentioned, previously and as we've discussed before, we continue our activities around business development, looking for late-stage assets that we can acquire in license that would add value to our pipeline, whether it be near-term value or a little bit later, say, Phase II. Again, looking for good value that we can add potentially with our technology as well and develop those products to potential commercialization.

So let me just turn briefly here and discuss some of the product attributes and then as well the trial that I've discussed a few times now in cardiovascular safety. So KP1077 actually consists of 100% of serdexmethylphenidate, also known as SDX. This is the prodrug that's 70% of the product in AZSTARYS. So something we understand, and it's been well studied. It's also already designated as a Schedule IV by the DEA.
When we look at the product candidate, what we like about this is there's already features and benefits that distinguish it from the alternative products. So this would be generics as well as modafinil and other products that could be used to treat idiopathic hypersomnia. And so one is, of course, the scheduling feature. Another is the fact that it doesn't have drug-drug interaction potential with contraceptives in antidepressants, which are actually used quite heavily in that population.
What we're trying to show here is the potential additional features and benefits that could be studied. So we have a hypothesis. We really believe there's great support for it. As we get greater tolerability, less side effects, less issues with cardiovascular effects, less issues with some of the issues that can happen with stimulants, we believe we're going to be able to provide a higher dose. The higher dose should be more effective.
We also believe we have our dosing regimen that's actually unique here, where a dose in the evening will start to work in the morning, providing that additional lift as it were in the waking hours. This is a major side effect issue symptom of IH. And then as the day progresses, the morning dose will be able to provide that relief from the other major symptom, which is brain fog. And we all know that methylphenidate is great in helping with cognitive abilities. And just given the severe detriment here at the brain fog, we believe that we'll actually be able to show a bigger difference. The product itself is, of course, eligible for fast-track orphan drug and breakthrough therapy designations and has solid IP through at least 2037.
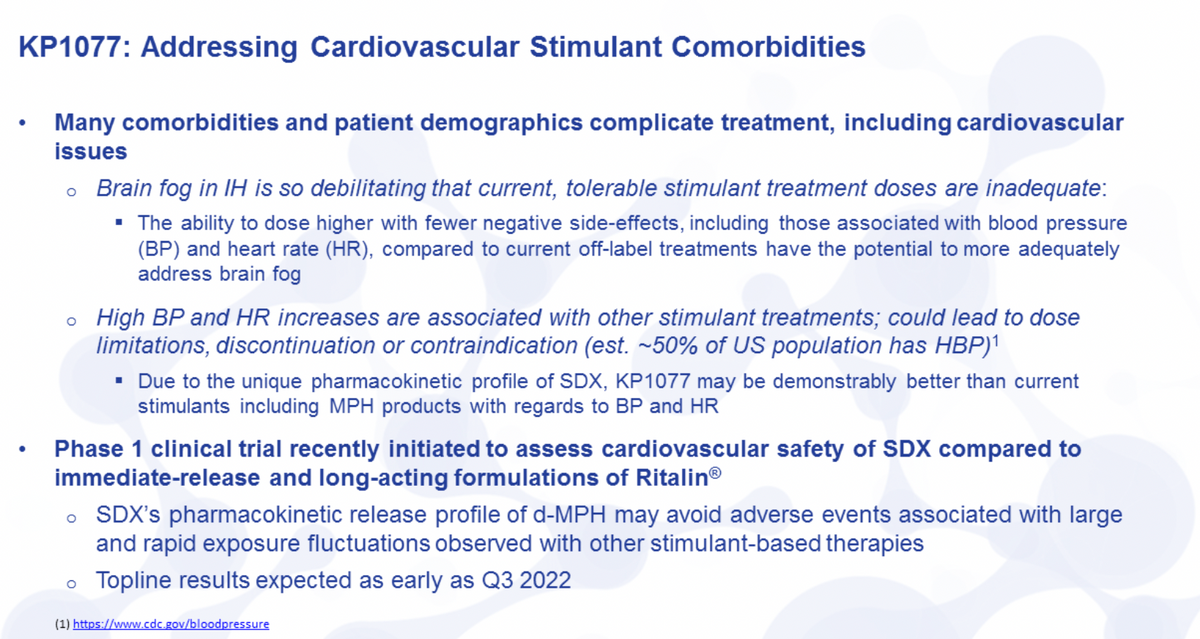
So specifically looking at the cardiovascular study and why we believe this is important. As I mentioned, brain fog is one of the most debilitating symptoms of IH. It's something that is inadequately addressed by current therapies, whether that's off-label -- well, it's actually off-label for most IH patients. The ability to actually dose higher to provide more methylphenidate, we believe is going to provide a greater effect and actually be more effective at treating brain fog.
The cognitive enhancement that you can receive with methylphenidate due to the neurostimulation has been well documented. It has its limitations in this population as you have a dose limitation, not just from a safety perspective, but from a side effect perspective, that physicians see. High blood pressure and heart rate are known to increase with stimulant treatments, and this has more to do with their immediate release or their double extended-release features.
SDX comes on very slowly under a nice gradual curve that increases to about a peak about 8 hours after dosing. And then again, comes down very slowly towards the end of that day. We believe that profile will allow us to dose higher and have less cardiovascular issues related to its dosing. We have a study that we initiated. It's ongoing right now. In order to assess that, cardiovascular safety, head-to-head with both immediate release and long-acting formulations of Ritalin®, these are the only 2 products that are actually approved to treat narcolepsy, a related rare sleep disease. And we should have top line results from that as early as Q3 this year.
So with that quick overview, LaDuane, I'll pass it over to you for a financial update, and then we will conclude with some upcoming milestones.


Thank you, Travis, and good afternoon. For Q1 of '22, we had revenue of $4 million derived primarily from consulting service fees. There were some royalties and then the success fee that we had reported out on from Corium related to our assistance in the approval of their product called ADLARITY®.
For this quarter, we had a net loss of about $1.9 million or $0.05 per basic and diluted share. And I would say, as we look forward to the rest of the year, as you would expect, R&D expenses will begin to increase quarter-over-quarter as the KP1077 program continues forward. Looking at the balance sheet, we remain in a very strong position with the balance at the end of the quarter of $119.1 million. And as we've noted in prior quarters, we remain confident that this extends our cash runway well beyond 2025. And so we have everything we need to execute our strategic plans as well as the development programs for 1077 that Travis has enumerated.
So with that, Travis, I'll turn it back to you.
Travis Mickle
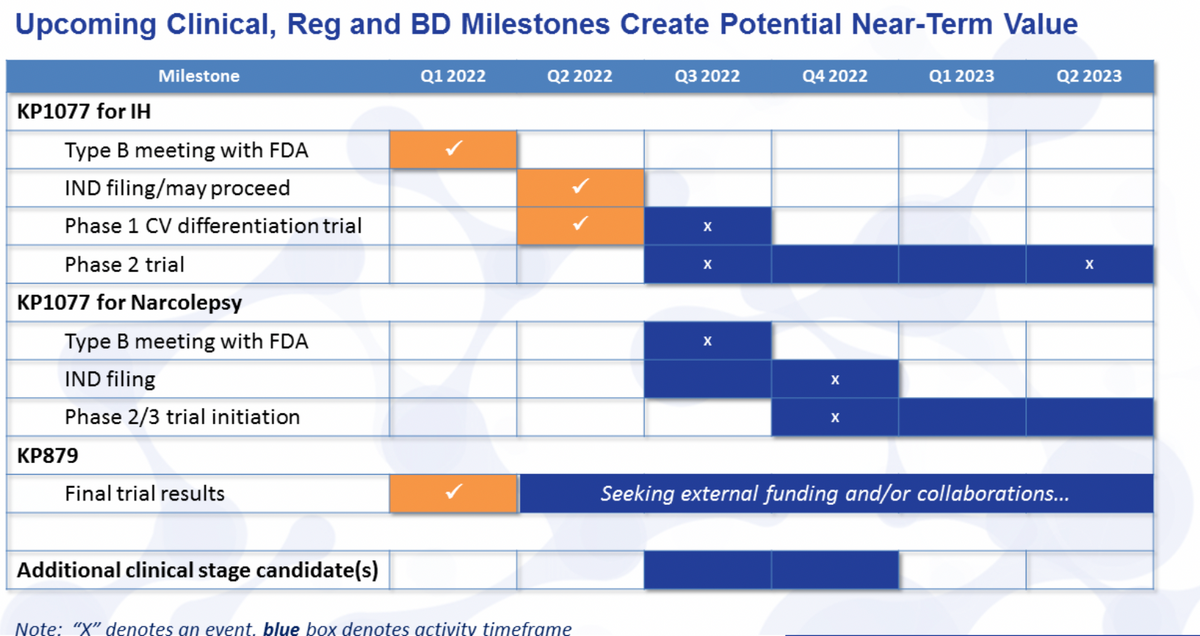
Thanks, LaDuane. So looking forward at the upcoming milestones for some of these we've already hit. Again, the Phase I trial, the IND filing that occurred this quarter, Type B meeting with the FDA in the first quarter. We should have, again, the data from the cardiovascular differentiation trial in the third quarter and be initiating the Phase II trial in the second half of this year. Shortly after that, of course, we would start the process within narcolepsy, though of course, we'll have the Type B meeting and the IND prior to that trial initiation.
We already had, and I didn't mention here some results on KP879, and we continue to look for external funding and collaborations. That's been part of our plan for that particular candidate. And then just briefly to remind everyone, we do expect the last half of this year to have some additional clinical stage candidates that we bring in through our business development efforts.
Again, I want to thank everyone for the time. I hope to continue to meet the progress that we've shown already this year and the last half of last year. And we'll continue to report an update as we have new information available. Thanks, everyone.